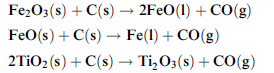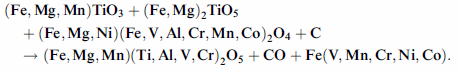_______________
Ilmenite ore Beneficiation and Upgrading
Excerpted from the following reference:
Mineral Processing & Extractive Metallurgy Rev.,28
: 1-58, 2007
Hemo-Ilmenite,Sulphate, and Upgraded Titanium Slags
By : Michel Gueguin & Francois Cardarelli
Technology Department, Rio Tinto Iron and Titanium, Inc,
Sorel Tracy, Quebec, Canada.
Copyright © Taylor & Francis Group, LLC
ISSN : 0882-7508 print/1547-7401 online
Ore Beneficiation
The chemistry and mineralogy of the titania-rich slag and its iron co-product is obviously related to the chemical and mineralogical composition of the hemo-ilmenite concentrate, and in a lesser extent, to that of the anthracite coal. Therefore, a brief description of the
ore is given hereafter.
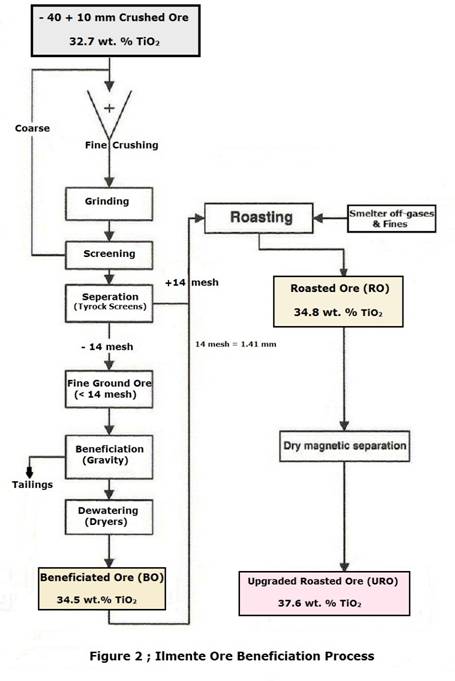
After common ore beneficiation techniques (e.g., comminution, sizing, and gravity separation), the run-of-mine yields a beneficiated ore (BO) with 34.5 mass percent TiO2
(Table1). A flowsheet of entire beneficiation process is presented in Figure 2 .
Microscopic examination reveals an intimate exsolution texture intergrowth of hematite Fe2O3
lamellae into ilmenite FeTiO3
grains. This kind of fractal microstructure indicates a progressive cooling of the parent magma from which originated the ore body. The
average mass fractions of ilmenite and hematite in the grains are 70 and 30 wt.%,
respectively (i.e., 69 mol.% and 31 mol.%). For that reason, the ore is usually called hemo-ilmenite. Other
obiquitous gangue minerals coming from anorthosite host rock, such as silicates,oxides,sulphides, and sulfosalts, are also present along with
hemo-ilmenite grains.
The major part of the silica,alumina, and calcia
present in the ore comes from gangue minerals such as plagioclase feldspar andesine with an average mineralogical composition that can be
expressed as a combination of anorthite (An=CaAlSi2O8),
Albite (Ab= NaAlSi3O8) and Orthoclase (Or=KAlSi3O8)
as An47Ab47Or6
.
Because the mixture of ilmenite
and hematite is so fine grained, an intricate comminution techniques cannot liberate the two phases. The beneficiated ore is then subjected to an oxidative
roasting to allow:
·
The oxidation of sulphide and sulfosalt minerals for the removal of most of the sulphur
content.
·
The formation of ferromagnetic domains within hemo-ilmenite
grains, to allow their magnetization in order to render easier their separation from residual gangue minerals.
Actually, hematite and ilmenite
are antiferromagnetic minerals and the roasting step induces atomic reordering within the
ilmenite and hematite crystal lattices that modify their magnetic behavior. For instance, there is aregion
in the ternary phase diagram FeO-Fe2O3-TiO2
(Figure 1), where the ilmenite-hematite becomes ferromagnetic.
During roasting conducted in air, the ore is treated at a temperature ranging between 900oC and
1000oC. The hematite reacts with part of FeO from the ilmenite to produce magnetite Fe3O4
and pseudobrookite Fe2TiO5 according The theoretical chemical reaction :

In practice, the actual composition of the hemo-ilmenite must be considered in the raosting
equation and the actual reaction can be described according to the following scheme :

From the above reaction scheme, it can be seen that at the end of the roasting process, the excess
ilmenite
is the major mineral phase along with hematite,magnetite,and pseudobrookite. The averaged empirical of the excess
ilmenite is (Fe1.080Mg0.129Mn0.004Cr0.002V0.002)Ti0.890O3,Which is close to that of the original ilmenite in the
beneficiated ore. As expected,the ilmenite
grains still host most of the manganese and magnesium while traces of vanadium and chromium comes mainly from tiny inclusions of hematite.
The magnetite appears at boundaries of ilmenite-hematite forming large rims around ilmenite with the empirical formula:
(Fe0.968Cr0.010Ti0.007Mg0.004V0.003Al0.003Ni0.002)3O4;
while the remaining hematite grains exhibits the formula:
(Fe0.791Ti0.135Mg0.024V0.005Cr0.004Al0.0035Mn0.001)2O3.
From these empirical formulae we can clearly see the pathways followed by minor elements during solid-state
reactions. For minor elements originally in solid solution into the hematite lattice such as Al3+,V3+, and Cr3+ along with some magnesium and titanium coming from the
ilmenite
grains, they follow the Fe3+ into the magnetite grains. Actually, the spinel structure - (The
spinel
are any of a class of minerals of general formulation A2+B23+
+ O42- which crystallize in the cubic isometric- crystal system)- accommodates a wide variety of mixed valence
metal cations (AB2O4 with A = Fe2+,Mg2+,Ni2+, and B = Fe3+,Cr3+,Al3+,V3+,Mn3+,
and Co3+). Therefore, the newformed
magnetite mineral in the roasted treated ore can be seen as a solid solution between pure magnetite an minerals having the
spinel
structure, such as magnesioferrite MgFe2O4,ulvospinel FeTi2O4, hrcynite FeAl2O4, Coulsonite
FeV2O4, manganese ferrite NmFe2O4, trevorite NiFe2O4, and cobalt ferrite CoFe2O4 .
Moreover, all sulphides
and sulfosalts are oxidized during roasting giving-off suiphur dioxides (SO2),
which is later absorbed into the off-gases scrubbers while most of the cobalt and nickel accommodate the spinel
structure of the magnesite, as mentioned previously. During roasting, most of the plagioclase feldspar along with
phlogopite
mica and spinel remain unaffected, as indicated by their empirical formula : Na0.515Ca0.450K0.480Si2.575Al1.414O8
and K0.965(Mg2.013Fe0.484)Si2.712Al1.384- O10(OH0.927)2
.
After roasting, the roasted ore is subjected to a magnetic separation using rare earths magnets that remove
most ore from the nonmagnetic gangue materials, specially plagioclases, spinel, and phlogopite
mica that form the black tailings.
The resulted upgraded roasted ore (URO) is then transported into the electric arc Furnace. The mineralogy of the
titanates
phases existing in the upgraded roasted treated ore are obviously the same as those existing as confirmed by the empirical formulae of
ilmenite
(Fe1.072Mg0.121Mn0.004V0.003Al0.001)Ti0.898O3, of magnetite (Fe0.968Cr0.010Ti0.007Mg0.004V0.003Al0.003Ni0.002)3O4
and hematite grains (Fe0.8143Ti0.107Mg0.017V0.006Cr0.003Al0.002Mn0.001)2O3,
except the fact that most of the gangue minerals are now removed.
_________________
Added notes about ternay diagrams :

Note : The composition of an iron-titanium oxide mineral can be illustrated
graphically on the ternary oxide diagram (Fig.1), the corners of which represent the minerals rutile,wustite, and hematite. The proportion of these 3 oxides in a mineral define a point on
the ternary diagram. The vertical distances of the point above FeO-Fe2O3
baseline reflects the amount of titanium in the lattice. Hematite is in a higher state of oxidation than wustite;
hence the horizontal position along the FeO-Fe2O3axis expresses the degree of oxidation.
The most important magnetic minerals belong to two solid-solution series : (a) the titanomagnetite, and (b) the titanohematite
series. The minerals of a third series, pseudobrookite, are paramagnetic at room temperature.
They are quite rare and rare of minor importance in rock magnetism. The compositions of naturally occurring forms of
titanomagnetite and titanohematite
usually plot as points on the ternary diagram that are displaced from the ideal lines towards the TiO2-Fe2O3
axis, which indicates that they are partly oxidized.
__________
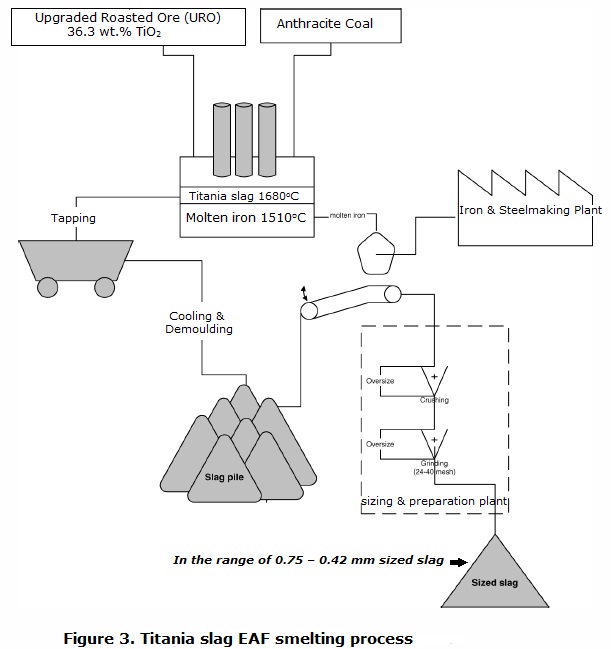
Upgraded roasted ore smelting
During the electrothermal reduction of
the upgraded roasted ore with anthracite coal as carbonaceous reductant
into an EAF using a transferred arc, the reduction takes place rapidly (Fig.3).The strong reducing conditions ensure that iron oxides values
are almost completely reduced to metallic iron while titanium dioxide TiO2 is rapidly reduced to titanium
sequioxide Ti2O3. All these reactions evolve carbon monoxide CO according to the following reaction
schemes :
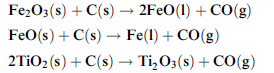
If we consider the initial chemical composition of the
hemo-ilmenite
ore, the chemical composition of the Soreslag as tapped, and the sensible and latent heats required to bring the feed materials at the operating
temperature, the theoretical specific energy consumption of the smelting process is about 0.903 kWh per kilogram of ore. Due to the
inescapable trace of moisture and the volatile matter (VM) of the anthracite coal, some hydrogen forms and combines to the carbon monoxide
to yield the smelter gas (i.e., CO + H2). At the high temperature existing in the furnace, iron forms dense liquid droplets (ρ = 6800 kg/m3) that sink by gravity settling to the bottom
of the furnace, forming a pool of a molten iron-carbon alloy. While a thick layer of titania-rich slag floats due to its lower density above the molten iron bath.The most salient
properties of titania-rich slags are a medium density of 3500 kg/m3, a low
dynamic viscosity of ca. 30 mPa.s (30 millipascal.seconds = 30 centistokes= 0.3 Poise= 0.3 stokes= 0.03 kg/ms) quite
constant at a temperature above 100oC of superheat, and finally a high electronic conductivity of 6,500 S.m-1 .
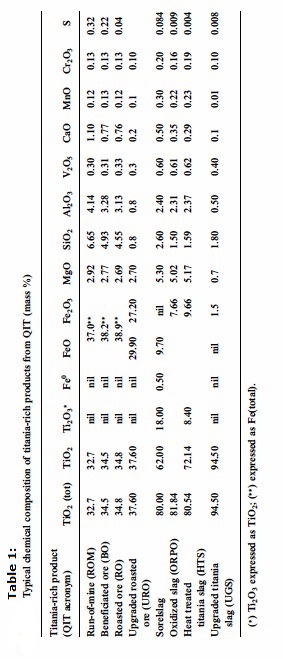
The titania-rich slag sold under the trade name Sorelslag® contains typically 80 mass percent TiO2 and exhibits a particular chemistry presented
in table 1. During slagging process, Ti4+ is partially reduced to Ti3+, as indicated by the
high Ti2O3 content of the bulk titania slag, while, reducible minor metal
cations such as V3+, Cr3+, and Mn3+ along with some Si4+ are reduced partially and are
distributed between the underlying molten iron layer and the titania-rich slag. Regarding the traces of sulphur coming from the anthracite coal , they combine with the volatile matter and the traces of moisture to give
hydrogen sulphide (H2S) and to a lesser extend carbonyl sulphide (COS) entrained with the smelter gas and are later trapped and removed by scrubbing. However, some remaining
traces of sulphur also distribute either into the titania-rich slag or into the molten iron. Therefore,
the molten iron has to be further purified by a proprietary QIT-injection process that removes all theses impurities prior to be converted
into high-purity pig iron (Sorelmetal®) or high-quality steel (Sorelsteel®).
Tapping and Cooling
The molten iron metal and titania-rich
slag are tapped at regular intervals from the EAF through distinct tapholes. The titania-rich
slag is poured directly into steel wagons lined with a crushed slag bedding. Afterward, the hot solidified slag
blocks are cooled under water sprays for several hours in order to build a thick protective skull. They are then
demoulded
and stored in the field until air-cooling ensures the complete solidification of the inner core.
During air cooling, the exposed surface of the slag block
undergoes charachteristic disintegration or spalling
due to chemical, thermal, and mechanical effects. This complex mechanism was early called decrepitation- (Decrepitata : to cause crackling until crakling ceases - by
cooling or heating)- in the late 1970s at QIT and later by others.
Based on the electron microprobe analysis and
th x-ray diffractogram, the major titanate
phase found in the solidified Sorelslag exhibits the karrooite structure- (is a model that describes cation ordering and stability
of pseudobrookite-type MgTi2O5 as a function of temperature and pressure)- and has the average chemical formula (Fe0.197Mg0.271Mn0.004)(Ti1.094Al0.041V0.0075Cr0.0025)2O5.
Moreover, past studies conducted on the Sorelslag
using x-ray absorbtion near edge spectroscopy (XANES) confirmed that chromium exists as Cr3+ in
Sorelslag
and vanadium is also assumed to exist in the trivalent oxidation state or even lower valence states (i.e., V2+). Hence, we can
see that most of the titanium combines with most of the Mg2+ and remaining traces of Fe2+
to form a phase that also accommodates part of the minor metals of the ore such as Cr,V,
and Al. The carbothermal reaction can then be expressed schematically as follows, highlighting the behaviour
of minor and trace metals:
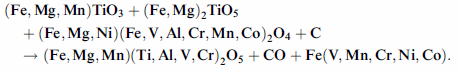
At this point, it is important to briefly describe the
pseudobrookite
group and its structure, which is of particular interest for understanding the mineralogy and chemistry of titania-rich slags. Minerals belonging to the pseudobrookite
group exhibit the typical concise empirical chemical formula MxTi3-xO5
(with 0 < x < 2). From a crystallochemical point of view, depending on the oxidation state of the mixed valence cations
and/or isomorphic substitutions, the crystal lattice can adopt the structure of one of the two end members; that is, either the
karooite structure MTi2O5
with M = Mg2+, Fe2+ or the pseudobrookite
structure senso-stricto- (means "in the stricter sense" ¹ sensu
lato means "in the wider sense)- originally described by Pauling (1930) M2TiO5 with M = Ti3+,
Fe3+, Al3+, V3+, Cr3+. Above 1320oC, it exists a contiuous solid solution series in the ternary diagram FeO-Fe2O3-TiO2 betwwen pseudobrookite Fe2TiO5,
ferro-pseudobrookite FeTi2O5, and anosovite Ti3O5
(see Figure 4)

Figure 4. FeO-Fe2O3-TiO2 ternary phase diagram
( reprinted by permission of the Mineralogical Society of America).
Moreover, solid solutions also exists in the MgO-FeO-TiO2
ternary diagram between anosovite (Ti3O5/ occurs in Ti-rich slags) , ferro-pseudobrookite, and the unnamed magnesium
dititanate (MgTi2O5) with an important mineral intermediate phase called armalcolite
(Mg0.75Fe0.25)Ti2O5/ or (Mg,Fe2+)Ti2O5 (see Figure 5)
Finally, regarding minor elements such as Cr, V, and
Al : they also substitute isomorphically to Fe3+ in the pseudobrookite
and yield the phase tialite Al2TiO5, berdezinkskiite
V2TiO5, and chromium pertitanate
Cr2TiO5. Therfore, the dual pseudobrookite-karrooite structures are of peculiar importance in the chemistry and mineralogy of
titania-rich
slags because 1) the crystallographic sites in the crystal lattice can accommodate a variety of mixed valence
metal cations and 2) the crystal lattice of the two end members, namely karrooite and
pseudobrookite, can easily self-reorganize into each other according to the change in the oxidation state of their mixed valence
cations and /or isomorphic substitutions.
Moreover, it is important to mention that
pseudobrookite
structures are metastable phases at ambient temperature. This behaviour was observed in
the laboratory for the pure synthetic phases such as Fe2TiO5
and FeTi2O5 that become unstable below 1140oC and 585oC, respectively (Grey and
Meritt
1981). According to Haggerty and Lindsley, pseudobrookite decomposes into rutile
and hematite below 585oC while ferropseudobrookite
decomposes below 1140oC into rutile and hematite (Haggerty an lindsley 1969).
This was also confirmed by in situ neutron and x-ray diffraction studies (Teller et al. 1990b). However, the high Ti3+ content of
titania-rich slag along with its content in minor trivalent metal cations
such as Al3+, V3+, and Cr3+ stabilize the structure of the pseudobrookite at room
temperature by forming solid solutions (Kesson and Lindsley 1975). A study performed on the thermal decomposition of Sorelslag shows that
magnesium also increases thr thermal stability of the ferro-pseudobrookite phase against further decomposition into rutile (Teller 1988). However,
studies involving Mossbauer spectroscopy indicated that the pseudobrookite
structure is distorted by these impurities.
Figure
5 : Mgo-FeO-TiO2 ternary system (reprinted by permission of
The
American Journal of Sience)
At that point, it is interesting to note that from 1950 until
1983, when the titania content of the slag was around 47wt.% TiO2 the major titanate
phase consisted mainly of armalcolite. A mineral discovered officially in 1969 on the
Traquility Base on the Moon and named after the acronym of the three astronauts of Apollo II; that is, N.A. Armstrong, E.E.
Aldrin, an M. Collins. Moreover, it can be seen that neither vanadium or chromium nor aluminium form distinct enriched titanates
and oxides in the titania-rich slag,but they are evenly dispersed into the karrooite phase. It is important to note that if mass fraction of vanadium and chromium in the
karrooite phasr are considered that is, 0.544 wt.%
V2O3 (i.e., 0.660 wt.% V2O5) and 0.182 wt.% for Cr2O3 This seems to
represent the minimum threshold concentration of these two deleterious chromophoric
impurities attainable for the Sorelslag, Below these limits, the smelting conditions or the chemistry of the ore
feed must be modified.
On the other hand, silica, alumina, and calcia
coming mainly from the feldspar plagioclases and to
a lesser extent from the ash content of the anthracite coal forms at the end of the cooling a glassy silicate phase containing traces
of titanium in the form of titanite CaTiOSiO4 with average empirical formula composition is Ca1.007Ti0.968Si0.995O5.
The slag also contains tiny globules of high-purity metallic iron
Fe with the averaged chemical composition Fe0.993Ti0.006. Because of their high purity, it was first suggested at QIT
in the 1970s and later supported and published (Toromanoff and Habashi 1984) that a redox
reaction occurs in the solid state between ferrous an trivalent titanium in the
pseudobrookite phase as :

While a smaller number of large micrometer-size globules of
metallic iron are also found in the solidified titania slag comes from the entrained molten iron during tapping of the
titania-rich slag. Actually, by contrast with tiny globules, they exhibit a high carbon content and traces
of other impurities, as indicated by a chemical composition Fe0.874Co0.002Ni0.001C0.117. In all
cases, either large or tiny iron globules always exhibits a rim of the rare sulphide mineral
troilite
FeS with the the averaged chemical composition (Fe1.007Cu0.002)S
that contains most of the traces of copper. This sulphide
rim around iron globule was also reported in Russian titania slag (Reznichenko
et al. 1981).
As mentioned in the introduction, part of Sorelslag® is sold
to white pigment titanium dioxide producers worldwide to be used as a low-iron and titania-rich feedstock in the sulphate process as a
substitute or in combination with ilmenite. In this process the titania-rich product is digested into sulphuric acid and
precipitated, washed, dried, and calcined to yield anatase or rutile pigment. However, since 1997, a part of the production of the
titania-rich slag is also further enriched using a QIT proprietary process. The novel process consists to produce an upgraded titania-rich
slag (UGS® ) to be used in the chloride route. This route is based on the carbochlorination of the titania for producing a titanium tetrachloride called "Ticle", which is
further oxidized to yield titanium dioxide. The scope of the UGS process is to increase the titanium dioxide content
an
consequently to lowe the level of deleterious elements such as magnesium, calcium, and iron and to a lesser extent aluminium that strongly affect the efficiency of the carbochlorination process. In order to achieve such enhancement, the titania-rich slag is
crushed, sized, oxidized, reduced, acid leached, washed, and finally calcined.The
chemistry and mineralogy of the intermediate products are described hereafter to understand the relationship existing between the
Sorelslag
and the end product; that is, the UGS. The flowsheet
of the UGS process is depicted in Figure 6.
Sorelslag upgrading
Oxidation of Sorelslag
First the crude Sorelslag is
transferred to the sizing & preparation plant (SPP), where it undergoes crushing and screening. Afterward, the sized and ground slag
is directed to the oxidation-reduction plant (ORP). Sorelslag undergoes a thermal oxidation performed in
the oxidizer at circa 1000oC. The aim of this step is to oxidize all the Ti3+ into Ti4+ to render the
titanium insoluble as titanium dioxide (TiO2) during the subsequent high-pressure acid leaching. During this step all other
reducible cations are obviously oxidized to their higher oxidation state such as Fe3+, V5+, and Mn3+,
while the Mg2+,Ca2+,Al3+, and Cr3+ remain obviously unchanged.
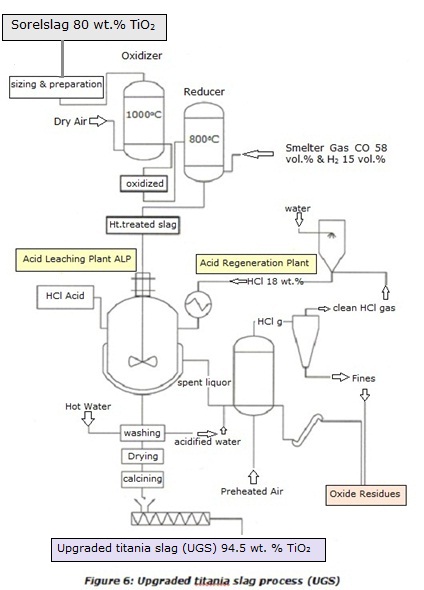
The pseudobrookite reacts with oxygen to yield some pseudorutile Fe2Ti3O9; (Fe0.195Ti0.452V0.013Mn0.0055Cr0.005Al0.073)Mg0.510Ti3O9 and ferrain rutile (Ti0.994Fe0.004V0.002Al0.001)O2
according to the reaction of oxidation :

These two phases constitute mostly the oxidized slag existing
from the oxidizer. Regarding the minor elements such as V5+, Cr3+ they mainly accommodate the
pseudorutile phase because they form minerals like schreyertite or kyzylkumite with the formula V2Ti3O9
and olkhonskite Cr1.5V0.5Ti3O9
that form solid solution with pseudorutile. Hence, they are evenly dispersed into the crystals and no single phase
containing these chromophoric elements was observed in all the studied samples.
Reduction of Oxidized Slag
The oxidized
titania-rich slag undergoes a thermal reduction at 800oC ensured by smelter gas (i.e., 85 vol. % CO15
vol. % H2 ). The reducing conditions existing into the reducer allows to reduce back the iron oxides into ferrous
oxide while titanium dioxide remains unaffected. The resulting product called Heat-Treated Slag
(HTS) consists mainly of a mixture of rutile (Ti0.993Fe0.001V0.005Al0.001
Cr0.001)O2 and ferroan karrooite (Fe0.178Mg0.266Mn0.006)(Ti1.100Al0.040V0.0075Cr0.0025)O5.
In addition, during the reduction process the iron
cations
initially contained into the iron-rich silicates tend to concentrate around pores, forming a thin micrometer-size layer and it also migrates
to the edges of the titanate phase forming a secondary ilmenite, which is not at all related to the ilmenite originally found in the ore. For that reason, it is called neoformed
ilmenite
or simply neo-ilmenite.
Moreover, we can also observe that minor
chromophoric elements mainly vanadium and chromium distribute evenly between the three phases, even if a slightly higher
concentration exists in the rutile
phase for vanadium. This dual thermal oxidation/reduction process is mandatory because it greatly enhances the acid leaching of impurities
contained in the original slag with minimal consumption of hydrochloric acid values and minimal loss of titanium dioxide and without
degradation of the original particle size.
__________
Added note :
Thermal Treatment of Titania Slag under Oxidation-Reduction
Conditions
T.A.I. Lasheen1
and M.E.H. Shalabi2
1Nuclear Materials Authority, P.O.Box 530 El Maadi, Cairo, Egypt
2Central Metallurgical Research and Development Institute, Cairo,
Egypt
Abstract
The present work provides a method for beneficiating of an Egyptian titanium slag by a thermal oxidation and
reduction in a controlled gas atmosphere to obtain leachable slag suitable for use as a feed material in the production of TiO2
by chlorination process. The mechanism of that process has been studied and the proposed reactions are presented. The effect of oxidation on
the enrichment of titanium into rutile phase by blowing oxygen into the slag was studied at relatively high temperatures (900-1100°C) for different times (30-90 min). Heating of a fully divided slag under oxidizing conditions resulted in a major
portion of iron in the ferric state Fe3+ and titanium species in Ti4+ state. The results demonstrate that through
oxidation, the content of pseudobrookite and rutile phases increase. Reducing of the pre-oxidized slag was carried out
using hydrogen gas at 800°C for different period of times to convert the major portion of iron in ferric state
Fe3+ to ferrous state Fe2+. As a result of these treatments most of titania phases were converted into
rutile. Thereafter, the reactive ore was treated with aqueous HCl acid then sodium
hydroxide solutions to leach impurities to obtain a beneficiated product with an increased TiO2
content.
______________
High-Pressure Acid Leaching
The heat-treated titania
slag exiting the reducer after cooling is sent to the acid -
leaching-plant (ALP), where it is acid leached under high pressure with an azeotropic
- (a mixture of liquids that boils at a constant temperature, at a given
pressure, without change of composition) - solution of hydrochloric acid. During this step, a major part of leachable cations such as iron,
magnesium, aluminium, manganese, calcium, vanadium, and chromium are removed from the product and dissolved into the acid as metal
chlorides or oxichlorides (eg., FeCl2, MgCl2, AlCl3, MnCl2, VOCl2, and CrCl3).
The leached titania-rich slag is then
washed, dried, and calcined to yield the so-called upgraded titania slag (UGS) with at least 94.5 wt.% TiO2
and less than 1.1 wt.% MgO and 0.1 wt.% CaO. Because most impurities were removed during the leaching step, the upgraded titania-rich
slag is made entirely of rutile. However, examination under the electron microprobe reveals a slight difference in
chemical composition existing between the core and the outside rim of the rutile
grains. Rutile in the core of the grains exhibits the following averaged empirical formula Ti0.896Fe0.045Mg0.089Al0.024V0.006Cr0.002Mn0.002O2,
indicating that some impurities- mostly Fe, Mg, Al, and V are still hosted by the rutile lattice while the
rutile
forming rim exhibits a higher purity, as indicated by the empirical formula Ti0.993
Fe0.003V0.005Cr0.001O2. This can be easily understood by the fact that the hydrochloric acid has
to diffuse into the grains to leach out the impurities. The low silica content of the UGS comes from a remaining glassy silicate phase
filling the cracks of the rutile grains.This amorphous solid exhibits the following composition 93.5 wt.% SiO2,
2.9 wt.% Al2O3. 1.8 wt.% TiO2, 1.1 wt.% CaO, and 0.5
wt.% Na2O.
At the end of the process, the spent acid liquor that contains
all the metal impurities as chlorides is generated by pyro-hydrolysis in the acid regeneration plant (ARP).
During this process, the pyro-hydrolyzer is continuously fed with the spent acid and hot gases. The existing bed of oxides act
as seed material to promote the nucleation and growth of new crystals. The process generates micrometer-size beads of metal oxides that
crystallize around the previous seeds, and hydrogen chloride (HCl) leaves the reactor and is absorbed to tield a fresh hydrochloric acid.
Afterwards, the fresh concentrated hydrochloric acid is reused
upstream in the process with some make-up acid to balance the process losses and the solid oxide residues are continuously discarded. Due to
high magnesia, alumina, and ferric oxide content, the major mineral phases that form during the pyro-hydrolysis
belong to the spinel group with magnesioferrite
MgFe2O4 and hercynite FeAl2O4
as end-members. They usually exist as solid solution between these two end members as Mg(Fe,Al)2O4
(82 wt.%) while the excess of magnesia (9 wt.%) appears as free periclase MgO and the traces of sodium and potassium chloride form a mixed chloride (Na0.8K0.4Cl).
Most of the vanadium and chromium initially contained into the spent liquor are hosted into the above spinel
minerals because they also form a solid solution as coulsonite FeV2O4
and chromite FeCr2O4.
Conclusions
In this comprehensive study, based on
microchemical analysis combined to x-ray diffraction, we have shown that titania-rich
slags produced from the smelting of hemo-ilmenite ore with anthracite coal and related products exhibits a chemistry and mineralogy unique among other
natural and man-made materials. This remarkable difference essentially due the elevate titania content of the materials combined with the extremely high temperatures involved during the metallurgical
processing that leads to the formation of rare titanate phases. Most of them belonging to the pseudobrookite-Karrooite
group. The most important results and observations are as follows.
1.
The major phase in the Sorelslag exhibits the empirical chemical formula (Fe0.197Mg0.271Mn0.004)(Ti1.094Al0.041V0.0075Cr0.0025)2O5
with a karrooite type structure. This structure is of peculiar importance in titania-rich slags because its crystal lattice can accommodate a wide variety of mixed valence metal cations.
In the case of titania slag, the structure host most of the deleterious chromophoric
metal impurities, especially iron, manganese, vanadium, and chromium that always form compounds in solid solution into the
titanate phase and were never found as single rich titanate, oxide, or silicate phase.
2.
The major titanate
phase in the Sorelslag can be seen as a solid solution of various titanates (i.e., armalcolite, ferropseudobrookite,
tialite V2TiO5, and Cr2TiO5) all with the
pseudobrookite-karrooite structure and the excess titanium oxide, which is not chemically combined forms a nonstoichiometric oxide with the empirical formula Ti13O25
part of the Andersson-Magneli phases. This highly corrosion resistant compound is consistant
with the fact that it was also observed in the insoluble residue after digestion of Sorelslag in sulphuric acid and to a lessed extent to the good
electronic conductivity of the titania-rich slags
either in the solid or molten state.
3.
The removal of impurities from the Sorelslag by a proprietary
hydrometallurgical process yields the purest synthetic rutile on the market, which is suitable for the chloride
route for producing titanium dioxide pigment.
4.
Regarding minor elements, neither vanadium nor chromium were
observed as a single titanate or oxide V2O5
nor Cr2O3 in the QIT products, but they were always detected as solid solutions inside the other
titanate
or oxide phases.
____________
Added Topic ... Referring to the source mentioned in Fig.
7 :
The process is operated in the smelting furnace
semi-continuously. Ilmenite, reductant and electrical energy are discharged into the furnace as
contiuously
as possible. The operation is only stopped for adding electrodes, planned and unplanned maintenance, and for process-related incidents like
severe slag forming. Off-gas is extracted from the furnace continuously, while the slag and metal are tapped separately in batches.
Ilmenite and reductant enter the furnace through a single hollow graphite electrode that extends
down into the furnace through the centre of the furnace roof. Since the electride
is consumed as it delivers energy, it acts as an additional carbon source. Energy is discharged into the furnace via an open arc between the
tip of the electrode and the bath. The only other inputs into the process include air drawn in during periods of negative furnace pressure,
and water when leaks are present in the furnace roof , off-gas duct or other water-cooled elements.
Ilmenite is expected to melt quickly as it travels through the arc and into the slag bath. This due to
its relatively low melting point of 1379oC and its small particle size of between 100 and 1000
μm.
As reductant particles are heated up in the arc and slag bath, these particles devolatilise
(to remove or to cause volatile material) and possibly break up due to thermal chock. Reduction reactions are expected to take place at the
interface between liquid slag and reductant particles. The products of these reactions include gas (primarly
CO), slag (enriched in TiO2 and Ti2O3), and iron metal. Some reduction reactions also occur at the
interface between the slag and metal bath because of the high carbon content (around 2%) of the metal bath.The
products of these reactions are the same as for reduction with reductant particles.
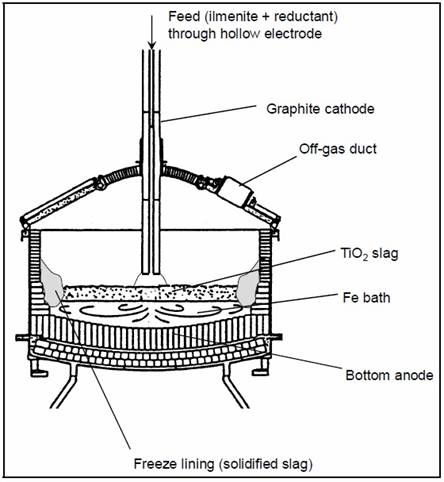
Figure 7 Schematic representation of a typical ilmenite-smelting DC Furnace
25 MW producing about 100,000 t/yr of high-grade titanium dioxide (TiO2) slag
Pistorius, 1999/ reprinted
from the Dr. degree Dissertation of Johannes Hendrik
Zeitsman
Because of the density difference between metal and slag, metal
collects at the bottom of the furnace as a metal bath and slag on top of it as a slag bath. Small metal droplets are present in the slag
bath due to the reduction reactions taking place there, but these droplets continuously find their way down to the metal bath or are
reabsorbed into the slag. Gas bubbles forming in the slag bath due to reduction reactions rise up through the slag bath and escape into the
furnacr freeboard.
Metal is extracted from the furnace through a metal tap hole. The
slag tap hole is situated at a differene angular position than the metal tap hole, and higher in the furnace wall
than the metal tap hole. This is because the slag bath is floating on top of the metal bath. Off-gas, fumes and dust that continuously rise
up into the furnace freeboard are extracted by the off-gas system through a duct installed in the furnace roof.
The off-gas system is operated in such a way that a slight
positive pressure is maintained in the furnace freeboard. This is done to prevent air from being drawn into the furnace. Air consumes carbon
and oxidizes slag, resulting in reduced reductant efficiencies and electrode wear.
Process Inputs
Ilmenite: Ilmenite is a primary input material into the furnace, having particle size usually less than 1000 μm. In some cases it is roasted to increase its magnetic susceptibility over that of unwanted impurities such as
chromium bearing spinel (nominally FeCr2O4) and garnet [(Fe2+.Mg.Mn.Ca)3(Al.Fe3+)2Si3O12].
Such roasting is usually oxidizing and could result in some of the ferrous iron being converted to ferric iron. The end result is some Fe2O3
in an M2O3 solid solution with FeTiO3 together wit TiO2 as a separate phasr.
Reductant: The reductant
used in ilmenite smeltingis
firstly chosen to have low ash content because virtually all of the ash reports in the slag phase as impurities. Secondly, low volatile
matter content is preferred. This to ensure a lower rate of gas evolution from the reductant that can contribute to slag forming foaming without significantly contributing to reduction. Anthracite and
charred coal (charcoal- coke and charcoal are both produced by charring) are therefore mostly used as
reductant.The particle size of the reductant is chosen relatively fine, less than 10mm. A fine particle size is beneficial for reaction rates in the
furnace due to the increased surface area. Too-fine particles are not favoured
because they can blow out of the furnace with the off-gas and reduce reductant efficiency.
Graphite Electrodes:
The graphite electrodes used in the Dc ilmenite-smelting furnaces are pre-baked, consisting of more than 99% graphite. The amounts of
moisture, ash and volatile matter in these electrodes are negligible. They are manufactured with a hole in the centre through which feed
material enters into the furnace.
Process Outputs
Titania Slag:
The Furnace is operated with a slag bath temperature of around 1700oC. Slag is therefore tapped from the furnace around this
temperature. The target total TiO2 content (all Ti reported as TiO2) of the slag is around 85%. The viscosity values
of the slags are reported to be around 0.03 kg/ms above their liquids temperature. This is several orders of magnitude less than the
viscosity (106 kg/ms) of SiO2 at its melting point.
Note : 0.03 kg/ms = 30 mPa.s
/ The SI derived unit for dynamic viscosity is the pascal
second 1 pascal second is equal to 1Kg/ms, or 1000 millipascal.second.
Pig Iron:
Heat is extracted at a significant rate from the metal bath through the hearth and sidewalls. For this reason the temperature
of the metal bath can be up to 150oC lower than that of the slag bath. The carbon content of the metal is around 2%. It also
contains other elements such as Si,Mn,S and P. The levels of such elements in the metal tend to increase as the level of
reduction of the slag is increased.
Off-Gas : The major species in the off-gas include CO (estimated at between 80%
and 90%) as a product from the reduction reactions, and H2 (estimated at 5 to 15%) from volatile in the
reductant. Some CO2, H2O, N2 and SO2 are also found. Because of the high CO and H2
content, this gas is rich in chemical energy. For this reason it is recycled for purpose such as ilmenite
pre-heating.
Dust and Fumes:
It is likely that thermal shock is experienced by materials as they enter the zone of the electric arc and the bath just beneath it. This
can cause reductant and ilmenite particles to break up, producing fine dust particles that are carried
into the furnace atmosphere. This is a likely reason for the thick dust cloud present in the furnace freeboard during normal operation. In
addition to this, species like Mn and siO2 are fumed off at the operating temperatures of the furnace. The dust extracted from the furnace
by the off-gas system is enriched in these species.
Energy : Energy leaves the furnace via a number of routes. Liquid slag (at around
1700oC).
Liquid metal (at around 1600oC) , and off-gas and dust (at around 1700oC) carry out large
quantities of sensible heat. In addition to this, heat is lost through the hearth, sidewalls and roof.
__________
http://wmr.sagepub.com/content/24/1/74.full.pdf (Added)
There are four major companies in the world, other than
Russia, engaged in the recovery of TiO2 from ilmenite (Mukherjee1998) by the slag route (Table 1).
QIT-Feret Titanate Inc. of Canada, a subsidiary of the RTZ (Rio Tinto-Zinc) corporation produce slag and iron at its Sorel
plant by smelting rock ilmenite analysed as 34% TiO2 and53% iron oxides in
its maiden electric furnace. The plant has incorporated roasting and beneficiation processes for the upgrading of the ore, thus producing
1.05 million tonnes of sulphatable slag analysed as 72% TiO2. The company also produces 0.2 million
tonnes
of upgraded slag analysed at 95% TiO2, 0.6% Al2O3, 1.95% SiO2, 0.14% CaO, 0.6% MgO, 0.05% MnO, 0.46% P2O5, and 0.03% Cr2O3.
Richards Bay Minerals (RBM) of South Africa employs four rectangular six-in-line graphite-electrode furnaces for the smelting of
ilmenite. Each furnace is 19 m long, 8 m wide, and has a power supply rated at 105 MVA (each pair of electrodes being supplied by a
35 MVA transformer) (MacPherson1982, Skillen
1992) Each of these three-phase open-arc furnaces is rated at 69 MW. The process technology, originally developed
by Quebec Iron & Titanium (QIT Fer et Titane) of Sorel, Canada, was supplied to RBM in the mid-1970s, with the first furnace starting up early in 1978. The
fourth furnace started up in mid-1991. The process has been adapted to smelt fine ilmenite obtained from a beachsand
deposit on the north-eastern coast of South Africa.
An open-bath approach is employed for which careful control is
required to avoid erosion of the refractories of the sideand end-walls by the very reactive titania slag. The installed electrical capacity possibly makes these furnaces the largest scale AC transferred-arc
plasma operation to date. RBM is currently the only South African producer of titania slag, and has an annual production capacity of some 2 million tonnes
of ilmenite (FeO.TiO2) and 125 000 tonnes of rutile (TiO2).
The ilmenite is smelted to produce about 1 million tonne of slag and 550 000 tonnes
of pig iron per annum (Robinson1992). RBMs ilmenite
is of too low a grade to be used directly for the production of pigment or synthetic rutile. Therefore, RBM
followed the slag-beneficiation route, and currently produces about half of world titania slag output.
Tinfos Titan and Iron KS of Norway produces sulphatable
slag from hard rock ilmenite analysed
at 44% TiO2 and 46.5% FeO. The process based on AC smelting technology, is slightly different from
QIT/RBM which involves additional steps such as pelletization and pre-reduction at 1150°C prior to smelting.
Presently, about 0.3 million tonnes of slag analysed at 7585% TiO2 and 0.1 million tonnes
of pig iron are produced per year.
Namakwa Sands of South Africa in collaboration with MINTEK successfully commercialized DC smelting technology for processing sand ilmenite
containing 47.3% TiO2 and 46.7% iron oxides.
Viscosity of the slag plays a major role during smelting of
titanium-containing ores. In this context the effect of slag composition on the viscosity of the melt during the smelting operation is
discussed by Ross (1958). It was found that titanium sesquioxide (Ti2O3) and titanium monoxide (TiO) increased the viscosity
of both acidic and basic slags. High slag viscosity causes problems in working of the furnace and in handling of
the slag. An increase in viscosity also occurs due to the formation of titanium carbide or titanium carbonitride, which are formed during carbothermic
reduction at high temperatures. The presence of titanium carbide and carbonitride in the slag provides resistance towards leaching efficiency of titanium. Hence, the formation of these
compounds is not desirable during smelting.
Therefore, by controlling the operating conditions, namely the
reducing environment, leachable slag containing lower oxides such as Ti2O3 or TiO can be obtained, but that will lead to various other problems as mentioned above.
To obtain leachable slag, formation of titanium carbide or
carbonitride
and a large proportion of titanium dioxide should be avoided. To avoid the formation of titanium carbide, reduction of TiO2 in
the system to Ti during smelting has to be controlled, because the presence of metallic titanium will lead to the formation of
TiC as per the following reactions:

Table 1: Metallurgical process options for beneficiation of ilmenite
to titania slag.
|
Process |
Description |
Plant status |
|
QIT Electrosmelting |
Carbothermic smelting of hard rock ilmenite at1700°C to pig iron and
sulphatable titania slag (8687% TiO2)
|
SOREL Quebec1 000 000
tonnes year1 slag
|
|
RTZ Iron & Titanium Electrosmelting
|
Same as above: but customized to suit beach sand
ilmenite
|
Richards Bay, South Africa, 1000 000
tonnes year1 of slag and 500 000 tonnes year1
pig iron
|
|
Submerged arc smelting process |
Pre-reduction of ilmenite (50%
TiO2), followed by smelting in arc furnace to produce pig iron and
titania slag (87% TiO2)
|
TINFOS, Norway, 200 000 tonnes
year1 of slag and100 000 tonnes year1 of pig iron
|
|
Plasma DC arc smelting technology (South Africa Anglo
American Corp.)
|
Carbothermic smelting of ilmenite in DC arc plasma furnace to pig iron and
titania slag
|
NAMAKWA Sands Ltd., South Africa, 1 100 000
tonnes year1 of slag and 450 000 tonnes
year1 of pig iron ISCOR, South Africa 220 000 tonnes year1
of slag from 1999
|
|
Upgraded slag technology
|
Conditioning of the SOREL slag and its leaching followed by
calcination to UGS(up-graded slag) (95% TiO2)
|
SOREL, Quebec 200 000 tonnes year1
of slag to be expanded to 600 000 tonnes year1
|
As titanium metal cannot exist in the presence of iron oxide, the
formation of TiC can be avoided by keeping a small amount of FeO in the slag. This can be achieved by smelting ilmenite and deficient carbon to have TiO2, Ti2O3,
TiO and a relatively higher concentration of FeO at equilibrium in the melt. Further reduction of FeO, if needed, could be achieved by slow injection of additional carbon.
It has been reported (Miller 1957) that rapid cooling by water
quenching causes slag to have a small grain size resulting in the formation of fairly large amounts of TiO2, which is insoluble in
sulphuric
acid. Block casting and slow cooling of slag produced better results. In this operation the slag is poured into large moulds, which are
slowly cooled by spraying water inside the quenching chamber. Slow cooling of slag resulted in the formation of larger grain size and the
formation of a smaller amount of insoluble TiO2, which may give a comparatively higher leaching efficiency.
The results of solubility tests show that when the TiO2
content of ilmenite and its alteration products goes over 60%, the solubility decreases below the value generally accepted (98% TiO2
solubility) by the pigment industries. Acid-soluble slag with TiO2
content around 90% has been produced from ilmenite. It has been suggested (Sinha 1979)
that to achieve the required high degree of solubility, the titanium originally present in the feed material has to be converted to the
anosovite
phase. Anosovite is a solid solution structure based on Ti3O5. High-temperature oxidation of ilmenite using the Beecher process developed in the Government Chemical Laboratories of Western Australia (Mackey 1994)
produces a pseudobrokite structure, which upon solid state reduction with carbon forms anosovite, minor
sub-rutile and metallic iron.
Several companies (Mackey 1994) are currently using thermal
reduction of ilmenite to produce pig iron and titania slag. All the Fe2O3 and
FeO is reduced to metallic iron with a small amount of iron in the slag. A
pseudobrokite
phase is produced which is suitable feed for the sulphate
process. Therefore, further reduction of the slag having pseudobrokite
phase may yield the anosovite phase, which is suitable for titanium recovery.
A lot of literature is available on the generation of high titania slag, its quality and leachability. However, due to the high power consumption,
India just has started to exploit the indigenous raw material on a commercial scale. Therefore more research and development efforts are
needed not only on minimization of the power requirement but also on the study of the slag quality and its physical properties and
leachability. Most of the processes developed for titanium recovery from slag are still at laboratory scale. These processes have to
be tested and transformed into commercial-scale operations using the indigenously produced raw material.
Conclusions: The following conclsions can be drawn:
-
Smelting of various types of ilmenite results in slag that is rich in TiO2.
-
In smelting, the desired slag chemistryand phase can be obtained by the proper control of the process
parameters (i.e., temperature, reductant, time, flux, etc.).
-
A clear seperation of slag and metal can be achieved by controlling the viscosity.
-
The rate of cooling of the slag is an important factor in achieving the desired leachability of the slag.
-
Detailed charachterization of the slag is essential to understand the leaching behaviour.
__________
The following data and information are excerpted from :
Techno-Economical Feasibility Report on Constructing in the ARE, an Experimental Plant for
Concentration and electrometallurgical processing of Ilmenite Ores / Vol.1-
USSR,Moscow 1971.
-----------------------------------------
In late 1970 Titanium Institute and the Zaporozhie Titanium
and magnesium (USSR) works have conducted pilot-scale testing of ilmenite
smelting of an ore about 400 tons of concentrate, were processed using furnace with a power 5000 kva. The figures
obtained from the testing will be used as the basis for estimates of techno-economical feasibility report on constructing of an experimental
plant for production of titanium slag and pig iron (excerpted
from page 16 of the above mentioned report).
Investigations have shown that ore practically was not concentrated and in this
connection further metallurgical testings were carried out with crude ore. From the starting ore there
have been produced titanium slag with TiO2 content up to 70-77% and pig iron. This slag can be directed to titanium
dioxide pigment production by sulphate
process - (chromium dioxide is no more than 0.05-0.08%, and phosphorous is no more than 0.019-0.047%). As to its content the slag is
analogous to the Canadian slag (Sorelslag), which contains up to 70-75% TiO2 and is processed into
titanium dioxide pigment by sulphate process.
Pig iron should be subjected to refinement from sulphur and
phosphorous, as the content of these elements in such amounts (0.3-2.5% and 0.6-0.8% respectively) makes it (pig iron) unsuitable for
direct use. It can be assumed that for the purpose of more rational and deep refinement from sulphur the ore should better be subjected to preliminary roasting in rotary furnaces. (excerpted from page 18 of the above mentioned report).
An ore experimental smelting furnace of 5 mva (megawatt)
capacity is taken as a main unit . This furnace design is at present the most experimentally operated and
practised.
Accepted process factors and marketable products output by the experimental plant for Abu
Ghalaga Ore are given in the following Table :
|
Name
|
unit
|
Data and outputs
|
|
Specific consumption of electric energy
Per 1 ton of slag
|
Kwh
|
2000
|
|
Furnace capacity as to slag
|
t/year
|
14450
|
|
Recovery into marketable slag
|
%age
|
96.5
|
|
Plant capacity as to slag
|
t/year
|
13900
|
|
Pig iron production
|
t/year
|
9950
|
Pig iron produced as co-product contains a number of economic components (Ti,Cr, and V) . But its use presents difficulties because of higher
sulphur
content, as mentioned before, it can be assumed that for the purpose of more rational and deep refinement from
sulphur
the ore should better be subjected to preliminary roasting in rotary furnaces. (excerpted
from pages 22 and 23).
It is recommended that the starting raw material from Abu Ghalaga ilmenite deposit, to be in the proportion between oxidized and non-oxidized fraction. Composition of raw materials and
finished products is given in the following Table: (excerpted from 32)
|
Name
|
Chemical composition in wt.%
|
|
TiO2
|
Fe2O3
|
FeO
|
SiO2
|
Al2O3
|
MgO
|
MnO
|
CaO
|
Cr2O3
|
|
Ore
|
38.70
|
22.03
|
28.07
|
4.56
|
1.89
|
2.44
|
0.21
|
0.75
|
0.07
|
|
Anthracite
|
---
|
3.2
|
----
|
2.06
|
0.99
|
0.34
|
0.01
|
0.37
|
---
|
|
Titanium Slag
|
76.0¨
|
---
|
3.5
|
8.35
|
4.0
|
4.9
|
0.3
|
1.6
|
0.08
|
|
Pig Iron
|
0.25
|
96.64
|
---
|
0.31
|
----
|
---
|
0.14
|
---
|
0.05
|
Continued
|
Name
|
Chemical composition in wt. %
|
|
V2O5
|
ZrO2
|
S
|
P2O5
|
C
|
Volatiles
|
Balance
|
|
Ore
|
0.48
|
0.17
|
0.7
|
0.03
|
---
|
--
|
0.1
|
|
Anthracite
|
---
|
--
|
1.4
|
0.03
|
85.5
|
6
|
---
|
|
Titanium slag
|
0.33
|
0.34
|
0.56
|
0.02
|
---
|
---
|
---
|
|
Pig Iron
|
0.17
|
---
|
0.93*
|
0.01
|
1.5
|
---
|
---
|
¨- Total Ti reported as TiO2
regardless of valence state.
*- Pig iron composition is given when tapped from the furnace.
|
Name
|
Unit
|
Quantity
|
|
Total
|
Per 1 ton
Of commercial slag
|
|
I- Annual product output
|
|
1. Slag 76% TiO2
|
ton
|
13900
|
|
|
2. Pig Iron
|
ton
|
9950
|
0.716
|
|
II- TiO2 Recovery from the ore
|
|
1-
Into slag
|
%
|
96
|
------
|
|
2-
From slag being tapped
into commercial slag
|
%
|
96.5
|
-----
|
|
3- Complete Recovery
|
%
|
92.64
|
-----
|
|
III- Main Material Consumption
|
|
1- Ore 38.7 TiO2
|
ton
|
29330
|
2.11
|
|
2- Anthracite
|
ton
|
4450
|
0.320
|
|
3-
Graphitized electrodes
|
ton
|
330
|
0.024
|
_____________
The following data and information are excerpted from :
Technical Proposal for Contract for Development of Feasibility
Report on Production of Titanium Slag and Metal from ABU-Ghalaga Ilmenite Ore Deposit/ All Union Research And Design Titanium Institute/ USSR July 1991.
·
Anthracite used as reductant in smelting process containing : 81.7% C, 2.18% S, 0.004% P, 3.56 % moisture, 9.18%
ash, 3.32% volatile.
·
It should be noted that slag and metal final composition show a little dependence on the
use of oxidized or non-oxidized ore.
·
Slag composition will also show a little dependence on the method of smelting :
batch one stage, or continuous two stage.
·
The furnaces used for smelting slag and the method of smelting are determined by the
pre-set annual slag productivity. With annual capacity of furnaces up to 30,000 ton the most expedient way is to carry out smelting by a
batch process using 5-6.5 MVA furnaces; from 30,000 to 50,000 t annually, a batch process with 16.5 MVA furnaces; 80,000 to 100,000 t
annually, two stage continuous process with 16.5 MVA furnaces and rated transformer power up to 25 MVA.
The production of titanium slag by a batch process comprises
the following technological operations:
·
Transportation, preparation and batching of ore and coal.
·
Charging, melting and production of conditioned melt of slag and metal in ore-smelting
furnace.
·
Slag and metal tapping through
through separate tap-holes into ladles.
·
Slag casting in conveyor casting machine, cooling and crushing.
·
Metal refining as for sulphur, refinement as for pre-set elements, casting in conveyor machine into commercial ingots.
·
Purification of technological gases in scrubbing system.
Process material flow for batch process are given in Figure
8 .
Consumption coefficients per 1 ton of slag (9%
FeO) :
·
Ore quantity
. 1.95 t .
·
Anthracite quantity
.. 0.228 t .
·
Electrodes consumption
.. 33 kg .
·
Metal yield
. 597 kg .
·
Electric energy consumption
.. 2480 Kwhr.
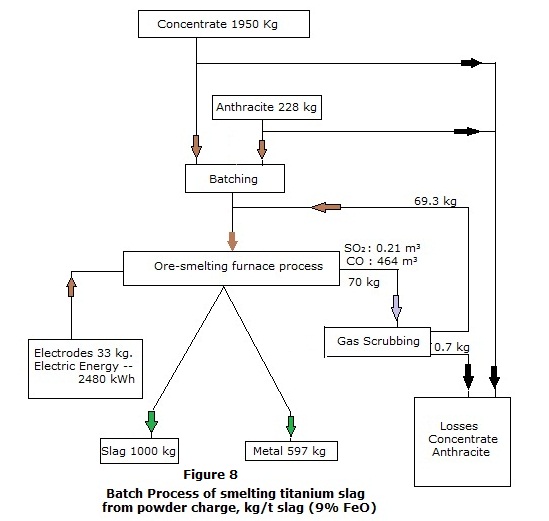
The production of titanium slag by a continuous process
comprises the following technological operations :
·
Transportation, batching of concentrate, coal, bentonite.
·
Grinding the concentrate and coal , mixing them with bentonite.
·
Preparation of pellets
·
Reducing Roasting of pellets in rotating furnace.
·
Melting of pellets in ore-smelting furnace.
·
Batch tapping of slag and metal through separate tap-holes into ladle.
·
Slag casting in conveyor machine, cooling and crushing.
·
Metal refining as for sulphur, refinement (when necessary) to pre-set chemical composition , casting in conveyor machine into commercial ingots.
·
Purification of thnological gases in scrubbing system.
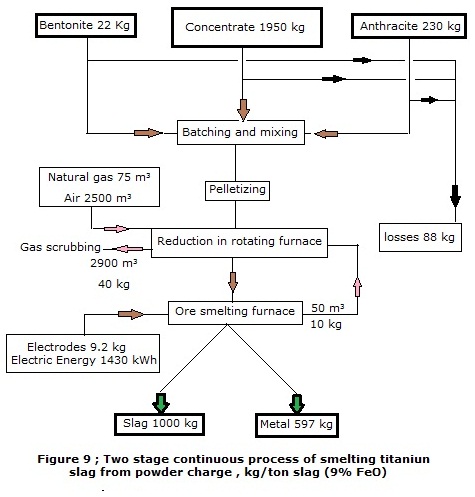
Process material flows are given in Figure 9 .
Consumption coefficients per 1 ton of slag (9%
FeO) :
·
Initial ore quantity
.. 1.950 ton.
·
Antharacite (reductant) total consumption
0.288 ton.
·
Electrodes consumption
.. 9.2 kg.
·
Bentonite consumption
22 kg.
·
Metal yield
. 597 kg.
·
Electric energy consumption
1430 kWhr
The proposed flow of the process of continuous slag melting uses bentonite powder as a binder. In the world practice it is the most common and available type of binder.
The researches carried out in Titanium Institute (USSR 1991) have
shown the feasibility of smelting titanium slag suitable for production of titanium dioxide pigment by sulphate
process, and by chloride process from the Egyptian ore of Abu-Ghalaga deposit, the content of titanium oxides in
slag amounts to 70% & 77% respectively in both processes, and that of FeO amounts to 10% & 3% respectively in both
processes. The following table shows mass fraction components %ages by wt. in slag and metal in both cases :
|
Suitable for sulphate route of pigment production (10% FeO by wt.)
|
|
Titanium
slag
|
TiO2
|
FeO
|
Fe metal
|
SiO2
|
CaO
|
MgO
|
Al2O3
|
MnO
|
P2O5
|
|
70.17
|
10.0
|
1.05
|
8.50
|
2.62
|
3.11
|
5.85
|
0.20
|
0.004
|
|
Pig
iron
|
Ti
|
.
|
Si
|
.
|
Mn
|
.
|
Cr
|
.
|
P
|
|
0.36
|
0.5
|
0.016
|
0.040
|
0.033
|
|
Suitable for chloride route of pigment production (3% FeO by
wt.)
|
|
Titanium
slag
|
TiO2
|
FeO
|
Fe metal
|
SiO2
|
Cao
|
MgO
|
Al2O3
|
MnO
|
P2O5
|
|
77.52
|
3.06
|
0.68
|
8.66
|
2.66
|
3.43
|
4.68
|
0.20
|
0.005
|
|
Pig
iron
|
Ti
|
.
|
Si
|
..
|
Mn
|
..
|
Cr
|
|
P
|
|
o.26
|
0.5
|
0.043
|
0.035
|
0.031
|
Continued
|
Suitable for sulphate route of pigment production (10% FeO by wt
|
|
Titanium
slag
|
Cr2O3
|
ZrO2
|
V2O5
|
C
|
S
|
|
|
0.06
|
0.06
|
0.50
|
0.19
|
0.18
|
|
Pig
iron
|
V
|
|
S
|
.
|
C
|
|
0.030
|
0.18
|
3.90
|
|
Suitable for chloride route of pigment production (3% FeO by
wt.)
|
|
Titanium
slag
|
Cr2O3
|
ZrO2
|
V2O5
|
C
|
S
|
|
|
0.02
|
0.06
|
0.17
|
0.14
|
0.43
|
|
Pig
iron
|
V
|
.
|
S
|
.
|
C
|
|
0.031
|
0.18
|
0.92
|
The proposed technological operations and consumption
coefficients are preliminary ones and will be made more precise in the final variant, when Titanium institute developes feasibility report on producing titanium slags and metal from Abu-Ghalaga
ore deposit.
_________
Oxidation and Reduction roasting followed by leaching (see Fig.6)
____
Excerpted from
: Jacobus Philippus Van Dyk / Process Development for the production of Beneficiated Titania Slag- Submitted in
fulfillment of the requirements for the degree "Philosophia
Doctor"/ Faculty of Engineering- University of Pretoria.
__________
In 1996 a plant based on oxidation and reduction roasting
followed by leaching was commissioned in Canada by QIT for upgrading of Sorelslag®.
Sorelslag® is produced from Allard lake ilmenite that contains relatively high levels of alkaline earth impurities such as CaO and
MgO
(Compare with Abu-Ghalaga Ti Slag). The following table gives the chemical composition of
Sorelslag®.
Sorelslag® Composition (wt.%)
|
TiO2*
|
FeO
|
Al2O3
|
CaO
|
MgO
|
MnO
|
SiO2
|
Cr2O3
|
V2O5
|
|
84.8
|
3.76
|
3.62
|
0.47
|
5.89
|
0.28
|
3.06
|
0.027
|
0.65
|
(*Total Ti reported as TiO2 regardless of valence state)
Sorelslag consists mainly of the pseudobrookite
solid solution with a minor amount of glassy silicate. Pseudobrookite
is a solid solution of iron and titanium oxides with the general formula M3O5. The MgO
impurity is present mostly in the pseudobrookite phase, while the CaO
impurity occurs in the glassy silicate phase.
These phases are inherently inert towards the action of mineral
acids and this makes the slag difficult to upgrade. The upgraded slag (UGS) process modifies the phase composition of the slag to increase
the leachability
of the impurities.
The first step of the process consists of sizing the slag by
grinding, screening and classificationto the 75-850 μm
size range with a mean particle size between 250 and 350 μm. The slag is then oxidized in a fluid bed roaster at
1025oC for 1 hour. During oxidation all the Ti(III)-oxide in the slag is converted to Ti(V)-oxide and
the Fe(II) oxide is converted to Fe(III) oxide. These reactions can be represented by the following equation.

The oxidation results in a major rutile
(TiO2) phase and a minor pseudobrookite phase (M3O5). The glassy silicate phase
decomposes into wallastonite
(CaSiO3) and tridymite (SiO2). The decomposition of the glassy silicate phase is trigged by
the oxidation og FeO
and can be represented by the following equation:

Following oxidation the slag is reduced in a fluid bed roaster at
850oC for 1 hour. Reduction of the oxidized slag takes place in two stages. In the initial stage the Fe(III)
oxide is converted to Fe(II) oxide. In the second stage an MgO-enriched ilmenite-geikielite solid solution and a
MgO deficient residual pseudobrookite
phase and arutile phase are formed. These changes are accompanied by the creation of a large number of pores and
other defects in the crystal lattice.
Next the roasted the slag is cooled before it is leached with
18-20% HCl at 150oC in a pressure vessel for 7 hours. During leaching the impurities are removed to form soluble
chlorides leaving an upgraded residue.
The leah residue is separated from the spent leach liquor, washed and calcined
at 800oC to remove moisture and residual acid. The resulting upgraded slag is a granular product with TiO2 content
around 95 % TiO2. The following table gives typical composition of UGS.
Upgraded Slag Composition (wt.%)
|
TiO2
|
FeO
|
Al2O3
|
CaO
|
MgO
|
MnO
|
SiO2
|
Cr2O3
|
V2O5
|
|
94.9
|
2.47
|
0.46
|
0.06
|
0.67
|
0.03
|
1.69
|
0.06
|
0.35
|
The Motivation for upgrading Chloride grade
titania
slag
All the known slag upgrading processes have as their aim the upgrading
of sulphate grade titania slag to chloride grade titania slag for economic and environmental reasons. The main reason for upgrading chloride
grade titania slag to a synthetic rutile product (as proposed here) is also an economic one. The figure shown below gives the 1997 prices
for titaniferrous feedstocks.

Titaniferous feedstock prices
There is a $ 110 price difference between the price of chloride
grade slag (85% TiO2) and UGS (95 % TiO2). The reason for this price difference can be related to the quantity of
effluent generated by the chloride process when these feedstocks are used. A higher purity feedstock generates
less waste and this make it easier to comply with environmental regulations. Based on this analysis there appears to be a need for a
chloride grade slag upgrading process.
__________
Added
notes :
1997,
rutile and synthetic rutile
prices were approximately US$500/tonne and US$400/tonne respectively. The price for concentrated ilmenite (50
wt.% TiO2) was US$72/tonne (FOB prices), (http://www.claudiaforgas.com/Tiomin.pdf). Abu-Ghalaga
crushed ilmenite price same year $ 21 fob Abu-Ghosson.
The chloride process, a more environmentally friendly process
than the older sulfate process, produces over 60% of the worlds TiO2 pigment. The chloride process requires a feedstock with a high TiO2
content (generally >85%). Most natural sources of high TiO2 minerals such as rutile, are now exhausted, so
chlorinatable feedstock is manufactured from ilmenite, a common mineral generally containing around 50%
TiO2. Upgrading can be achieved either by electrosmelting, which produces titania slag, or by chemical processing to produce the>90% TiO2 material known as synthetic
rutile.
|
Commodity
|
Unit ptrice
|
2009
|
2010
|
2011f
|
2012f
|
|
Ilmenite (sulphate)
|
US$/t
|
70
|
80
|
90
|
90
|
|
Ilmenite (chloride)
|
US$/t
|
110
|
110
|
110
|
110
|
|
Synthetic Rutile
|
US$/t
|
470
|
484
|
498
|
498
|
|
Rutile -bulk
|
US$/t
|
540
|
556
|
573
|
573
|
|
Source: LME;IRESS;GSJBW Research Estimates
f..forecasted
|
Key costs for titania slag
production for 120,000 tpa slag plant are :
Source:
http://www.pyrometallurgy.co.za/InfaconXI/078.pdf
|
Ilmenite Feestock
(40% TiO2)
|
214,286 tpa
|
|
Power
|
1160 kWh/ton of ilmenite
|
|
Reductant
|
0.14 kg/ton of ilmenite
|
|
Electrodes
|
3.5 kg/MWh
|
|
Water
|
2KL/ton of ilmenite
|

-----------------------------
Related
Article:
Ilmenite upgrading by Murso Process
_________