Kaolin Wet-Processing
Prepared
by Eng. Atef Helal / May 4, 2012
Sources :Mainly SME
/ www.smenet.org / The Georgia Kaolins
Geology and Utilization – Copyright © 2002 & HAYDN H. MURRAY; Industrial Applicaions of
Kaolin; Georgia Kaolin
Company,Elizabeth, New Gersy & Hayden H. Murray 2007 / Applied
Clay Mineralogy (Occurrences,Processing and Application)
-------------
Kaolinite
is the proper name of the mineral about which this article is written, but in
industrial terminology the mineral is known as kaolin, so all references will
be to kaolin.
The
theoretical chemical composition of kaolin and a chemical analysis of a crude
kaolin from Dry Branch, Georgia, shown below, indicate the relative purity of
many of the Cretaecous kaolins in Georgia and South Carolina.

Kaolin has
many industrial uses. It is soft, has low viscosity at high solids content in
many systems, is readily wet and dispersed in water and some organic systems,
and can be produced with a controlled particle size distribution Some of the
important physical constants of kaolin are: specific gravity, 2.60; index of
refraction, 1.56; hardness (Mobs scale), 2; fusion temperature, 1850o
C; dry brightness, 78-92 percent,( measured at 458 mµ on a General Electric
recording speetrophotometer, against MgO Reference).
As mentioned
previously many kaolins have a low viscosity at relatively high solids content.
For example, many kaolins can be dispersed readily in water at 70 percent
solids by weight and the resultant slurry pours like fresh milk. This is an attractive
property for the paper coating industry.
The ranges of
particle size distribution in which kaolins are produced commercially are shown
in Fig. 1. Several end uses of kaolin depend upon the particle size
distribution. In many applications a coarse-particle kaolin may not work
whereas a fine-particle kaolin will, and vice versa. Some of the more important
industrial uses will be described in this paper, but it must be realized that
these descriptions, of necessity, are brief and incomplete. More than 2,000,000
tons of kaolin is used annually in the United States (de Polo, 1960, p.207).
Dry
Processing
Two basically
different processes are used to refine kaolins and remove the major impurities.
The simplest process is called air flotation or the dry process. The properties
of the finished product depend to a large extent on those properties inherent
in the crude kaolin. In the dry process operation, a deposit must be chosen
with desirable properties of color and relatively low content of grit ( > 44
µ). The crude kaolin is transported to the mill where the large chunks are
reduced to about egg size by roll crushers. The crushed kaolin is fed into
rotary driers and then into airfloating equipment. The latter usually consists
of a pulverizing unit and an air separator. The fine particles are transported
to collecting chambers and the coarse particles are fed back into the
pulverizer. Dry processing yields a product of relatively low cost.
Air- float
Kaolin are limited in their ablility to improve clay brightness or viscosity;
they typically shred,dry, and mill the crude clay while removing coarse grit
impurities on a moving column of air. Today air-float kaolin products make up
only about 2% of the total U.S. kaolin production value.

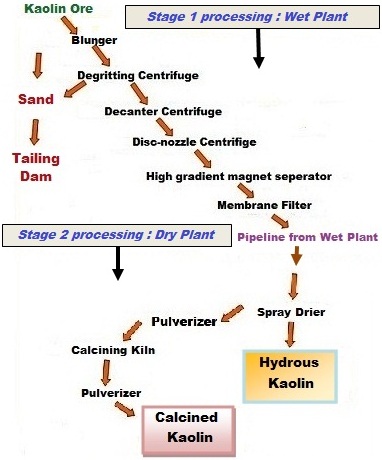
Wet
Processing
The second
process used to produce kaolins is much more complex and is called the wet
process. The kaolin is dispersed in water after it is mined. The first step
after dispersion is the removal of the coarse grit ( > 44 µ) by settling
procedures and vibrating screens. The resultant degritted slurry is fed into
centrifuges to separate the kaolin into fine, intermediate, and coarse particle
size fractions. These fractions can be chemically bleached to remove some
coloration caused by iron impurities. The kaolin is then dewatered through a
filtration process, dried in either rotary, apron or spray driers, and prepared
for shipment. This process is used to produce highly refined kaolins having
controlled properties.
Water-wash
processing of the crude clay, yields a product of considerably higher value,
and accounts for most of the kaolin produced in the Georgia district. One of
the main objectives of the water-wash process is to totally or substantially
remove all pigmentary impurity minerals that discolor the crude. Reductive
leaching and magnetic seperation are used to remove iron oxide mineral.
Iron-stained titanium dioxides are removed by magnetc seperation, selective
flocculation, and froth flotation. Organics are typically oxidized using ozone,
which breaks down the discolored organic molecules and makes pigmentary iron
oxides available for removal be reductive leaching. Complete removal of these
impurities is virtually impossible, largely because of the limitations imposed
by process cost.

§ Blunging
(high-energy mixing with water) to a dispersed 30-40% solids liquid slurry.
§ Degritting
to – 220 mesh (- 75 µm) to remove coarse sand and mica.
§ Blending
to achieve optimum mix of particle size, and to equalize variation of other
quality parameters.
§ Centrifugation
to product particle size ( 50% - 100%
< 2 µm).
§ Delimination
to reduce particle size, increase aspect ratio (ratio of diameter to
thickness), and improve brightness.
§ Brightness
improvement by one or more methods : reductive leaching, ozone oxidation,
magnetic seperation, froth flotation, or selective flocculation, foe example.
§ Filtration
to ~ 55% solids, spray drying or evaporation, repulpingto 70% solids slurry
or selling as dry-pigment product.
§ Optional
calcination to a higher brightness and more opaque pigment product.
Blunging
and Dispensing
The
process of converting run-of-mine crude kaolin to stable suspended slurry is
accoplished by mixing the kaolin with water and small percentage of dispersant.
Sodium hexametaphosphate (NaPO3)6, Sodium metasilicate (Na2SiO3 known as water glass or
liquid glass and produced according to the reaction: Na2CO3 +
SiO2 → Na2SiO3 + CO2), or organic colloid polymors are the most commonly used dispersants. The
clay may be mixed either in mobile blungers at the mine or in fixed blungers at
the processing plant. If the clay is blunged in the mine, it is transported by
slurry pipeline. The blunger is a high speed, high horsepower mixer which
breaks up the kaolin lumps into discrete individual particles.
A dispersant is necessary in order to keep the
discrete particles seperated from each other because otherwise the particles would
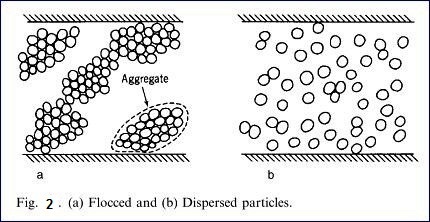
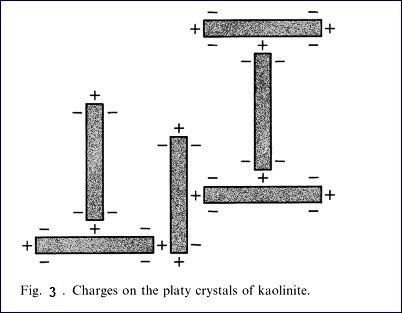
flocculate. Fig 2 is a diagram showing flocced particles and dispersed
particles. Fig. 3 is a diagram showing
the charges on the crystals of kaolinite. Because of the positive and negative
charges, the kaolinite particles are attracted and form large aggregates or
flocs. The addition of soluble dispersant which ionizes to produce cations that
are attracted to the negative charges on the clay particles so that each
kaolinite plate or stack has a similar charge and thus they repel each
other.The amount os dispersant added is quite small, of the order 4 – 12 Ib/ton
of kaolin, which is 0.2- 0.6 based on the dry weight of the kaolin.
Degritting (Grit
is defined as particles coarser than 325 mesh or 44 µm)
Coarse impurities (such as quartz, sand, muscovite
mica, and heavy minerals) are removed from the dispersed kaolin slurry during
the degritting step. One common method for removing grit is to pass the slurry
through drag boxes, which are known as
sandboxes. A residence time of about 30 min is adequate to allow the coarse
grit particles to settle to the bottom of the drag box. These coarse settled
impurities are then removed by drag slats and disposed of in waste
impoundments. Mica, which is flake shaped, does not settle as rapidly as the
quartz and heavy minerals so the slurry goes from the drag box to a vibratory
screen which removes the coarse mica and other floating debris that may be
present. Hydrocyclones are sometimes used instead of drag boxes, particularly
if the grit percentage is higher than about 15%. Hydroseparators are also used
to remove grit.

Crude Clay Blending
Typically, kaolin from several mines are blended in
mine or in processing plant terminal tanks to achieve the necessary quality. This
step when carried out in the mine by pumping the kaolin slurry from previous
step to a large holding tanks, which when filled and checked for
quality, is then pumped through a pipeline to terminal tanks at the processing
plant. The mine holding tanks are also used to blend kaolins in order to meet
viscosity and brightness specifications. The longest pipeline in Georgia is
about 35 miles (56 km) in length and the longest in Brazil is about 100 miles
(160 km) in length. Further blending, if necessary to meet quality
specifications, can be accomplished in the terminal tanks at the processing
plant.
The next
step in
the wet process (Fig. 4) is to fractionate the kaolin into coarse and fine
fractions. This is accomplished by continuous bowltype centrifuges,
hydroseparators, or hydrocyclones. After fractionation to a particular particle
size, the fine fraction and the coarse fraction of the kaolin are pumped to
holding tanks. The coarse fraction may be delaminated (which will be described
later) or is filtered and dried to produce filler clays. The fine fraction can
then be passed through a high intensity magnetic separator which removes
discrete iron and titanium minerals. Other processes used to remove the iron
containing titanium minerals, usually anatase, are selective flocculation and
flotation.
These
processes will be described later in this chapter. The fine fraction slurry can
go through one of the above processes before going to the floc and leach step
or it can go directly to floc and leach depending on the brightness of the
grade to be produced. The floc and leach step is to acidify and floc the slurry
at a pH between 2.5 and 3, which solubilizes some of the iron compounds which
stain the kaolin. Alum is sometimes used in combination with sulfuric acid to
give a tighter floc. At essentially the same time, a strong reducing agent,
sodium hydrosulfite, is added to the slurry to reduce ferric iron to ferrous
iron, which then combines with the sulfate radical to form a soluble iron
sulfate, FeSO4. The iron sulfate is removed in the filtration step,
which is the next step in the process. Quality control determines the quantity
of acid, alum, and hydrosulfate that is needed to give the best brightness
result.
After the floc
and leach process, the flocced slurry is pumped to filters to remove water and
the soluble iron sulfate. Usually water spray bars are used to wash the filter
cake to remove more of the iron sulfate. Commonly, the percent solids after the
floc and leach is around 25%. Large rotary vacuum filters or plate and frame
pressure filters are used to dewater the kaolin, raising the percent solids to
60–65%. After filtration, the filter cake is redispersed and pumped to a spray
drier where it is dried for bulk or bag shipments or the percent solids is
increased to 70% by adding dry spray dried clay or by large evaporators which
is the slurry solids necessary for most tank car or tank truck shipments. The
filter cake can be extruded and dried to make what is termed an acid kaolin
product.
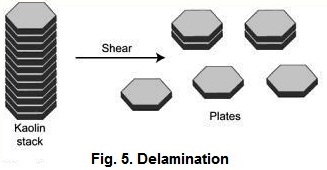
The coarse
fraction from the centrifuges is used either to make coarse filler clays or as
feed to produce delaminated kaolins (Fig. 5). The coarse thick vermicular
stacks and books of kaolin are pumped to delaminators which shears the plates
making up the stack or book into large diameter thin plates (Kraft et al.,
1972). These large diameter thin plates have what is termed a high aspect ratio
which is a ratio of the diameter to the thickness of the plate. The stacks and
books have a prominent cleavage, which is parallel to the (001) basal plane.
The coarse particles are cleaved by placing them in a baffled vessel filled
with media in which impellers strongly agitate the slurry. The spherical media
which can be used is wellrounded sand, alumina proppants, and/or glass,
plastic, zirconia, or alumina beads. This vigorous agitation of the media and
the coarse kaolin cause the kaolin to shear upon collision between the media
beads to produce a coarse delaminated plate with a high aspect ratio .
The magnetic separation process involves the use of powerful magnets with field strengths ranging from 2 to 6 T (Tesla is the international unit of magnetic flux intensity and equals to 10,000 gauss - cgs system of measure) . The range from 2 to 6T is achieved by using liquid helium cooled superconducting coils which results in considerable savings in electric power. The kaolin slurry is pumped through a highly compressed fine stainless steel wool matrix, which when energized, separates the magnetic minerals and allows the non-magnetic kaolinite to pass through the matrix. The magnetic field is periodically switched off so that the accumulated magnetic particles can be rinsed with water, thus cleaning the steel wool matrix. Fig. 6 is a diagrammatic representation of a 2 T magnet. The magnetic minerals that are removed are dominantly hematite and yellowish iron enriched anatase along with some ilmenite, magnetite, and biotite. The magnetic separation process was described by Iannicelli (1976) who was one of the first to advocate the use of magnetic separation in order to brighten kaolin clays. The development of high intensity wet magnetic separation for use in the kaolin industry has resulted in a huge increase in kaolin reserves which can be used commercially.

The froth
flotation process used to remove dark iron stained anatase which discolored the kaolin
was initially developed by Greene and Duke (1962). They used a calcium
carbonate carrier which was termed a ‘‘piggy back’’ process. Since then, the
flotation process has been improved so that now it has evolved into a standard
method in processing Georgia kaolins to make high brightness products of 90% or
higher. The dark iron stained anatase is selectively coated with a reagent
which causes it to adhere to air bubbles sprayed into the slurry. The air
bubble froth which contains the stained anatase rises to the surface of the
float cell and is skimmed off and discarded. Denver-type conditioners and float
cells are the most commonly used equipment. Recently, vertical column flotation
cells have been used which improves the separation of fine particles and also
increases product recovery. Most of the Georgia kaolins contain up to 2.5% TiO2
and by using the flotation process, the percentage can be reduced to as low as
0.3.
Selective
flocculation
is another process that can be used to reduce the TiO2 percentage. The process
was introduced in the late 1960s by Bundy and Berberich (1969) to produce high
brightness products of 90% or higher. Since its initial development, the
selective flocculation process has been continually improved and is now a
process which is used extensively to produce high brightness products (Shi,
1986, 1996; Pruett, 2000). This process is the reverse of flotation in that the
dark iron stained anatase is selectively flocculated so that it settles in a
hydroseparator while the kaolin remains suspended in a dispersed condition. The
flocculated anatase is discarded into waste impoundments.
Calcination
Another
special process used to produce value-added products is calcination, which was
introduced in the early 1950s. The kaolinite is processed to remove impurities
and a fine particle size gray kaolin is a preferred feed (Fanselow and Jacobs,
1971). The fine gray kaolin is spray dried, pulverized, and then fed to either
rotary or large hearth calciners and heated to as high as 1300oC.
The highest temperature of 1300oC is used to produce granules for
use in making refractory shapes and bricks. Most of the pigment grade of
calcined kaolin is heated to a temperature between 1000 and 1050oC. Shown
in the follwing reaction, the temperature at which the kaolin is dehydroxylated
to form metakaolin which is then transformed into mullite The metakaolin is an amorphous mixture of
alumina and silica that is used in several applications. The phase change at
980oC transforms the amorphous metakaolin into mullite (Al2SiO5).
This causes a significant increase in brightness and opacity which is discussed
later.

The hardness
of the calcined kaolin is about 6.5 on the Mohs scale, which is considerably
harder than the 1.5–2 hardness of hydrous kaolin. An 85% brightness feed to the
calciner will produce a product with a brightness of 91–93%.
Special
processes are used to modify the surface properties of kaolinite in order to
improve the functionality and dispersion of the product (Grim, 1962; Nahin,
1966; Libby et al., 1967; Bundy et al., 1983; Iannicelli, 1991). The
hydrophilic surface of kaolinite can be chemically treated to make them
hydrophobic or organophilic. These surface modified kaolins can then be used as
a functional pigment and/or extender in systems where the natural hydrophilic
kaolin cannot be used. The uses of these surface modified kaolins are discussed
in the following items.
Main Industrial Uses
Paper
The largest
single user of kaolin is the paper industry, which used approximately1,200,000
tons in 1958 (de Polo, 1960, p. 207). Because kaolin is used, paper products
print better and are made whiter and smoother. Kaolin used as a filler in the
interstices of the sheet adds ink receptivity and opacity to the paper sheet.
Kaolin used to coat the surface of the paper sheet makes possible sharp
photographic illustrations and bright printed colors. Kaolin constitutes nearly
one-third the weight of today's slick sheet magazines. The significant
properties of kaolin of greatest value to the paper industry are whiteness, low
viscosity, non-abrasiveness, controlled particle sizes, and flat hexagonal
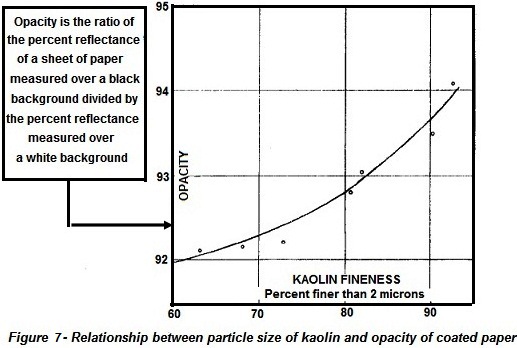
plates. Opacity is an extremely important property to the paper industry and
Fig. 7 shows the relationship between the particle size of the kaolin and
opacity. Brightness, gloss, and viscosity properties also depend on particle
size (Lyons, 1958, p. 81).
Flow
properties or rheology of kaolin clays, especially the kaolin coating clays
used in the paper industry, are very important because of their influence on
coat weight, smoothness, texture, and other properties. Figure 8 shows plots of
viscosity vs. shear rate for Newtonian, thixotropie, and dilatant fluids. These
three types of viscous flow are of primary interest to paper coaters. Particle
size distribution, particle shape, electrokinetic effects between particles,
presence of impurities and degree of flocculation and dispersion all affect
rheology. Rheology of kaolin slurries has been the subject of several papers
and patents in recent years (Albert, 1951, p. 456; Millman and Whitley, 1959;
Murray, 1961).

If one were
going to design in a laboratory a coating pigment for the paper industry, it
would be white, disperse readily in water, and have low viscosity, be soft, have
a fine particle size, and be a thin plate-shaped particle. Nature produced a
mineral which has essentially all the above properties, and that mineral is
kaolin. It can be readily seen why kaolin is an ingredient essential to the
paper industry.
Rubber
Kaolin is used
as a filler in many rubber goods. It adds strength, abrasion resistance, and
rigidity to both natural and synthetic rubber products. In general, most rubber
products extrude more easily after kaolin filler is added. The major reason
that kaolin is used in rubber compounds is its whiteness and low cost. Although
kaolin costs less than most other rubber pigments, it has excellent functional
properties.
Ceramics
Kaolin is used
in ceramic whiteware products, insulators, and refractories(Smoot, this
Volume). In whitewares, kaolin aids accurate control of molding properties, and
adds dry and fired strength, dimensional stability, and a smooth surface finish
to the ware. The excellent dielectric properties and chemical inertness of
kaolin make it well suited for porcelain electrical insulators. In refractory
applications, the dimensional stability, high fusion point, and low water
content, along with high green strength, make kaolin an important constituent.
Paint
Kaolin is used
in paint because it is chemically inert and insoluble in the paint system, has
a high covering power, gives the paint desirable flow properties, and is low in
cost.
Plastics
The addition
of kaolin to thermosetting and thermoplastic mixes gives smoother surfaces, a
more attractive finish, good dimensional stability, and high resistance to
chemical attack. In addition, the flat hexagonal kaolin plates hide the
reinforcing fibers and give the mix flowability to simplify tile molding of
complex shapes.
Other Applications
Kaolin has
many other industrial applications, some of which are listed here :
Ink, cement,
detergents, adhesives, fertilizers, porcelain enamels, insecticides, plaster,
paste, medicines, filter aids, roofing granules, food additives, cosmetics,
sizing, foundries, catalyst preparations, chemicals, bleaching, crayons,
linoleum, adsorbents, pencils, floor tiles, and textiles.
Special Applications Through Chemical
Modifications
Kaolin is
hydrophilic and can be dispersed in water and in some other systems. Because of
the nature of the chemistry of its surface, kaolin can be chemically modified
so that it will become hydrophobic or organophilic, or both. Generally, an
ionic or a polar non-ionic surfactant is used as the surfacetreating agent.
Eleetrophoretic
studies have shown that kaolin has an overall negative charge. The exchange
capacity of kaolin results from broken bonds and isomorphous substitution of A1
for Si in the tetrahedral sheet in the structure (Grim, 1953, p. 132). The
exchange sites are the locations on the surface where polar molecules can be
adsorbed and oriented.
The choice of
surface treatment or chemical modification depends upon the polarity,
structure, and composition of the organic system into which the kaolin is to be
utilized and the physical and chemical properties desired in the end product.
Wetting,
dispersion, flow properties, and general physical-chemical behavior are most
important in the organic medium into which the kaolin is to be utilized.
Thorough wetting of kaolin by the vehicle is essential in order to derive
maximum utility and functionality. Wetting breaks down the attractive forces
between kaolin particles and facilitates the coating of each particle with the
wetting medium. Even though wetting is complete, dispersion will not
necessarily be the ultimate, because the attractive forces between kaolin
particles may still be effective across interfaces causing loose agglomeration
of the particles.
Flow
properties are closely related to wetting and dispersion. Interparticle attraction
produced by unlike surface charges causes the formation of an internal
structure which inhibits flow and gives rise to thixotropy (Michaels, 1958, p.
26). Uniform wetting and good dispersion tend to give flow properties which
approach Newtonian and dilatant systems.
Improvements
in functionality of kaolin fillers after surface modifications have been noted
in the ink, paint, rubber, and plastics industries (Albert, 1960a, 1960b;
Bundy, 1961; Felletsehin, 1961; yon Volkenburgh, 1959: Werner, Marra, and
Gilman, 1960; Wilcox, 1961a, 1961b). Dispersion is essential for smooth films
with high finish and for improved color tone and texture. Decrease in viscosity
by virtue of improved dispersion enables higher pigment loadings as well as
easier workability.
0rganophilic
kaolins are being used in the rubber industry where it has been found that the
modified kaolin is easier to incorporate in the polymer system, higher pigment
loadings are obtained, and faster cure rates, higher modulus, and higher
tensile strength can be achieved. In paints, plastics, and inks, organophilie
kaolins are being used because they disperse and wet out rapidly, have better
suspension properties, and give superior water resistance and reduced
viscosities. In polyester resins, for example, some organophilie kaolins give
viscosities tenfold less than viscosities obtained for unmodified kaolins of
exactly the same particle size distribution and tested under similar
conditions.
The future
industrial use for surface-modified kaolins looks very good indeed. Additional
research will find new and better surfaetants for modifying the kaolin surface
and new end uses will be forthcoming. Industrial applications for
surface-modified kaolins will increase very rapidly in the next few years.
Calcined Kaolins
Another area
that deserves mention is the large industrial use of calcined kaolin. When
kaolin is heated to approximately 1050oC it is converted to mullite,
eristobalite, and/or a silica alumina spinel (Brindley and Nakahira, 1959, p.
312). This calcined product is whiter and more abrasive than the original
kaolin, and the surface chemistry and physical properties are completely
changed. The largest utilization of calcined kaolinsis in paint, rubber, and
plastics.
SUMMARY
Kaolin is a
unique industrial mineral that has properties that make it a functional
ingredient in many industrial applications. The largest tonnage of kaolin is
used by the paper industry. Surface-modified kaolins have a rosy industrial
future. Research efforts in this area are disclosing new and fascinating uses.
Calcined kaolins also are in demand particularly for paints, rubber, and
plastics.
---------------------------
End Note
: Delamination is the grinding or cleaving of kaolinite stacks into finer platy
particles. Delamination technology allowed producers to use coarse kaolin
stacks that had mostly been used as paper filler. The US Bureau of Mines in the
late 1950s developed attrition grinding of clay slurries with sand or other
media to delaminate and separate fine kaolinite platy particles. Delaminated
products show value in paper coating because they have good sheet coverage and
printability.
---------------------------
______________